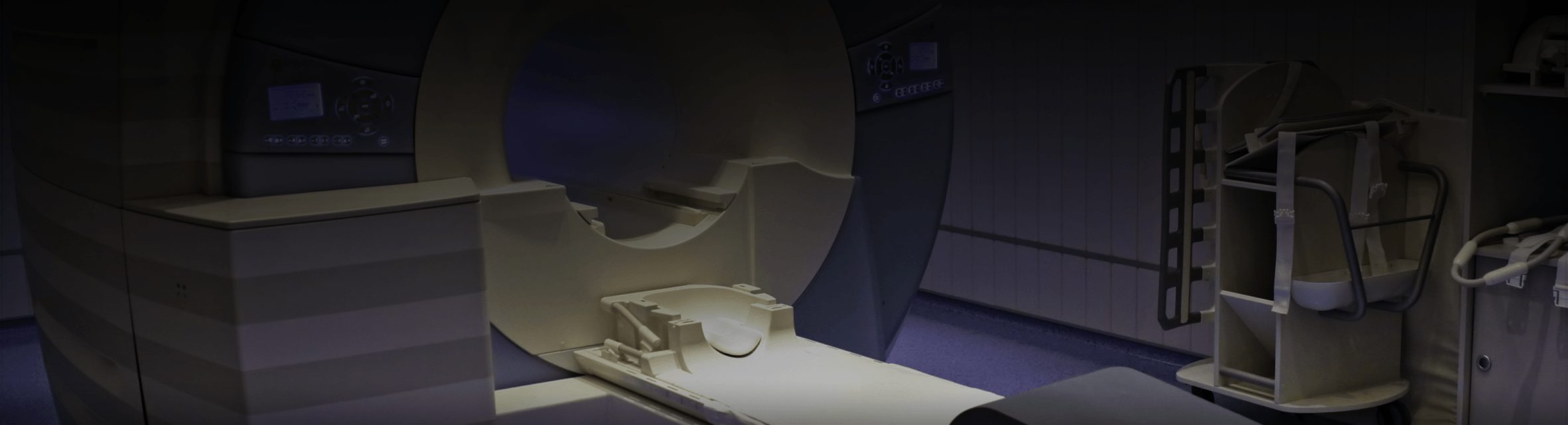
Our Medical Device Testing & Certification Capabilities »
Our thorough knowledge of industry performance and national safety standards and extensive global testing and certification resources, allows for swift and cost-effective market entry.
- Electromagnetic Compatibility (EMC) of Active Medical Devices
- Laser Safety Testing of Medical Laser Products Per IEC 60601-2-22
- FDA Submissions
- RFID Susceptibility of Active Medical Devices
- Certification Body (NCB) & Certification Body Testing Laboratory (CBTL) certification for Medical Electrical products
Why Us?
Through our vast network of testing laboratories, we are able to provide you with a true “one-stop shop” for your medical electrical device testing needs.
We can eliminate many of the headaches and hangups that occur when dealing with several different testing labs:
 |
Signing/executing multiple Non-Disclosure Agreements (NDAs) and all the associated red tape that ensues |
 |
Having and keeping track of multiple Points of Contact (POCs) to get information and updates |
 |
Facilitating and monitoring multiple testing schedules, and dealing with the domino effect if one schedule changes |
 |
Shipping your product to multiple locations, knowing where it is at all times, and hoping it gets from one place to the next |
We can provide the services you need and the peace of mind you want.
Our Services
Our Comprehensive Medical Device Testing Services comprise the following:
With more than 100 years of product safety and electromagnetic compatibility expertise, we can help you navigate complexities in medical device compliance. From testing & certification to global market access, we help you bring your product to market faster and more cost-effectively.
Testing Services:
- Product Safety Testing & Certification of Active Medical Devices
- Electromagnetic Compatibility (EMC) of Active Medical Devices
- Laser Safety Testing of Medical Laser Products Per IEC 60601-2-22
- FDA Submissions
- Battery Safety Testing of all Battery Chemistries used to Power Medical Equipment
- RFID Susceptibility of Active Medical Devices
- Certification Body (NCB) & Certification Body Testing Laboratory (CBTL) certification for Medical Electrical products
- Inspections of high-end medical equipment (e.g. MRI and CT scanners) in hospitals
- Accreditation Scheme for Conformity Assessment (ASCA)

CE Marking
In Europe, the use of a Notified Body (NB) is required in the approval or certification process for medical devices.
Our network of accredited laboratories and certification bodies offers a comprehensive range of testing and certification services for both active and non-active medical devices and in vitro diagnostic medical devices in line with the requirements of the MDR (Medical Device Regulation) for EU markets.
To assist you in placing your medical devices onto the EU market, Eurofins E&E has a number of Notified Bodies (NB) that can provide conformity assessment to the Medical Device Regulation (MDR) 2017/745 as well as to the In-Vitro Diagnostic Regulation (IVDR) 2017/746.
Medical Device Regulation (MDR) 2017/745
Our certification bodies in Finland (NB No. 0537) and Italy (NB No. 0477) are Notified Bodies under the MDR (2017/745) for both active and non-active medical devices.
In-Vitro Diagnostic Regulation (IVDR) 2017/746
Our certification body in Finland (NB No. 0537) is a Notified Body under the In-Vitro Diagnostic Regulation (IVDR) 2017/746.
Our EU Notified Bodies
Italy Eurofins Product Testing Italy Srl NB No. 0477
Finland Eurofins Electric & Electronics Finland Oy NB No. 0537
The current scope of accreditation for all EU Notified Bodies can be found on the EU Nando website.
Trust Your Testing to A Global Leader
 With more than 47,000 employees across more than 800 sites in 50 countries, we are a leading international group of laboratories providing an unparalleled range of testing and support services. Few testing laboratories can match the level of expertise, technological leadership, attention to quality, and customer service that have made us a global leader in the testing, inspection, and certification industry.
With more than 47,000 employees across more than 800 sites in 50 countries, we are a leading international group of laboratories providing an unparalleled range of testing and support services. Few testing laboratories can match the level of expertise, technological leadership, attention to quality, and customer service that have made us a global leader in the testing, inspection, and certification industry.
To learn more about our Comprehensive Medical Device Testing Services, click HERE to contact us, or email us at marketing@metlabs.com.
What is Medical Device Testing?
Medical device safety testing consists of a series of specific tests which are performed to certify that a product will not suffer from interference in the applicable medical environment and will prevent unacceptable risks to patients.
The manufacturer also performs a risk assessment and submits that assessment to the test laboratory in the form of a risk management document. This document provides evidence that a manufacturer has considered all potential hazards and mitigated associated risks to the highest degree possible.
The general medical device safety testing requirements for most electronic medical devices can be found inside the IEC 60601 standard family.